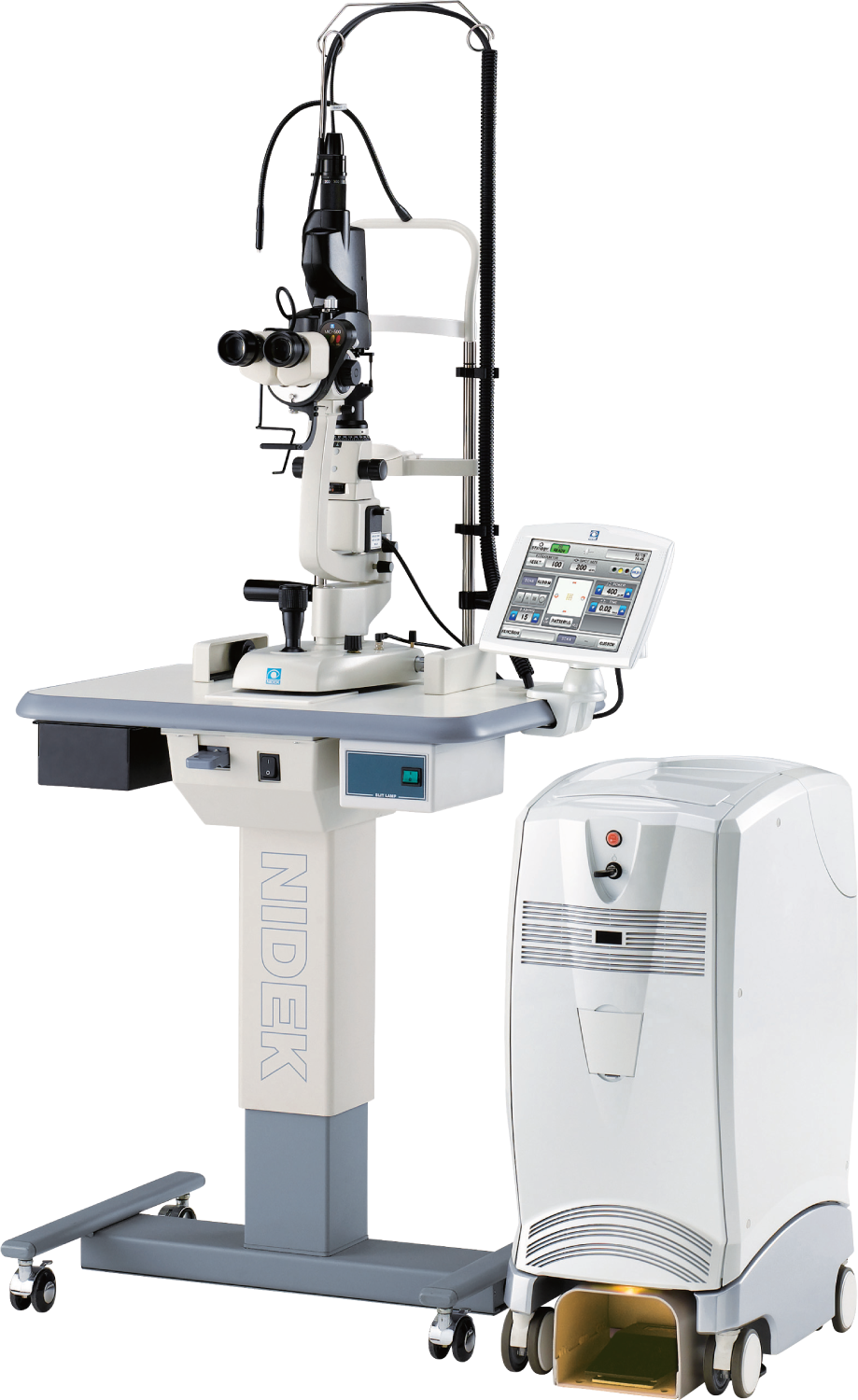
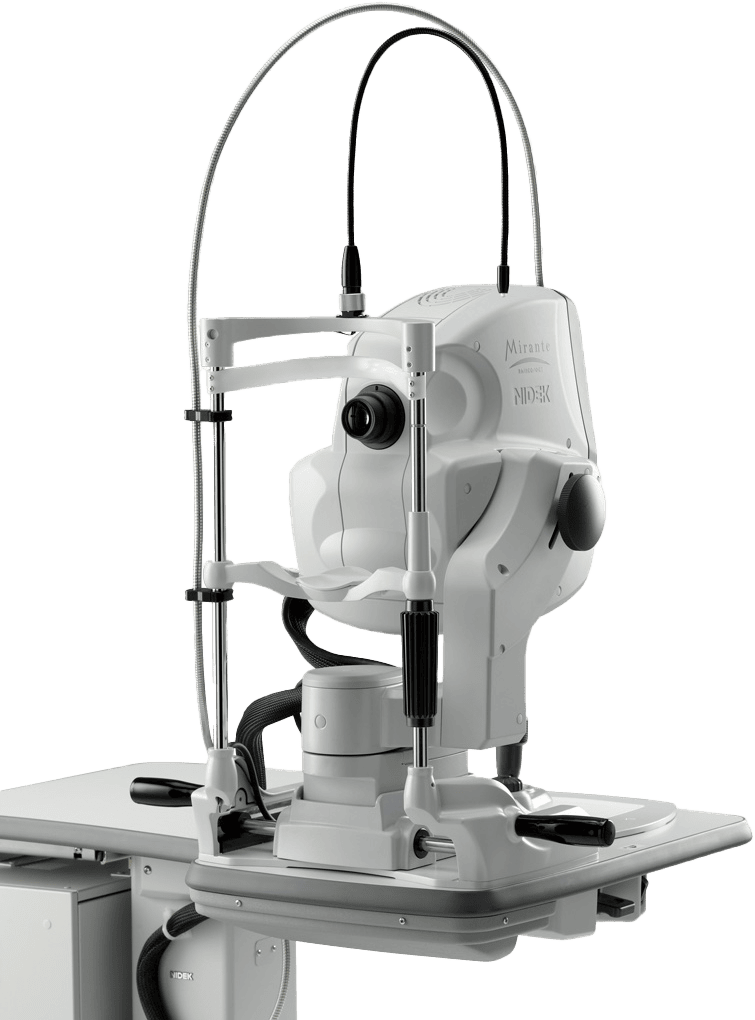
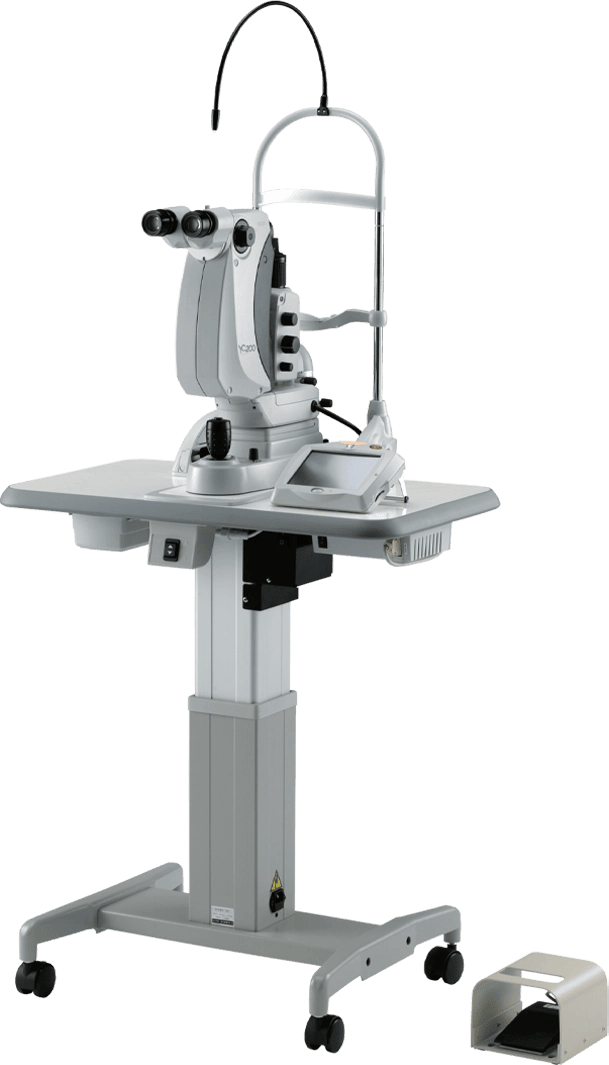
NIDEK launches in the USA, the Combination Delivery Unit for the YC-200 S plus / YC-200 and GYC-500 Green Photocoagulator as well as the Coaxial Illumination Tower for the YC-200 S plus / YC-200.
NIDEK is pleased to announce that the United States Food & Drug Administration (FDA) has issued 510(k) Clearance for the YC-200 S plus Ophthalmic YAG and SLT Laser System / YC-200 Ophthalmic YAG Laser System with GYC-500 Green Photocoagulator Combination Unit. With FDA clearance, the Combo YC-200 S plus / YC-200 is now commercially available in the USA. In addition, NIDEK is pleased to announce that the United States Food & Drug Administration (FDA) has issued 510(k) Clearance for the Coaxial Illumination Tower for the YC-200 S plus Ophthalmic YAG and SLT Laser System / YC-200 Ophthalmic YAG Laser System. With FDA clearance, the Coaxial Illumination Tower is now also commercially available in the USA.
Versatile Combo Lasers
The YC-200 S plus / YC-200 can be easily connected to NIDEK’s Green Laser Photocoagulator (GYC-500), allowing treatment of a wider range of patients and indications. Space requirements are minimized, and the optional combination adapter includes an illumination tower (split mirror illumination).
Coaxial Illumination Tower
The YC-200 S plus / YC-200 enables viewing and illumination of opacities in the vitreous with the optional coaxial illumination tower, which makes visual axis and illumination axis coaxial. The tower optimizes visualization during posterior membranectomy, capsulotomy and iridotomy, and illuminates vitreous opacities with the on axis/off axis illumination.
The YC-200 S plus / YC-200 is the advanced successor to the YC-1800 laser. The YC-200 S plus / YC-200 lasers build on the popularity and technology of the YC-1800 by incorporating newer optical designs, engineering and software advances to ensure precise targeting of pathology, while ensuring efficacious treatments and enhancing surgeon visualization of laser delivery.
About NIDEK
Founded in Gamagori, Japan in 1971, NIDEK continues to be a global leader in research and development, design, manufacture, and distribution of ophthalmic equipment. The United States subsidiary based in Silicon Valley, California, provides sales and service for ophthalmic lasers, refractive lasers, and many advanced diagnostic devices.
Contact:
NIDEK INC
info@nidek.com
Caution: U.S. Federal Law restricts this device to sale, distribution, and use by or on the order of a physician or other licensed eye care practitioner. Specifications may vary depending on circumstances in each country. Specifications and design are subject to change without notice.